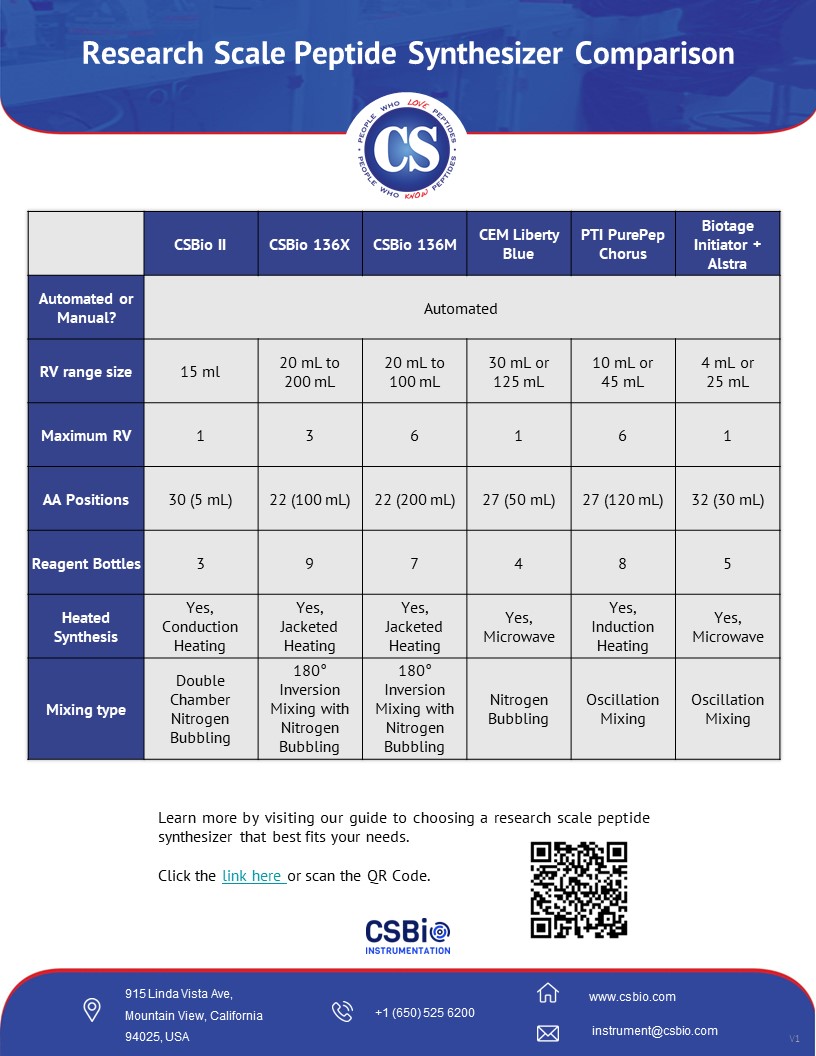
Research Scale Peptide Synthesizer Comparison - A Guide to Choosing a System that Fits Your Needs
We've gathered the key things to know to adequately compare the most popular synthesizers on the market for users searching for a peptide synthesizer.
Updated for 2022
Introduction
A major reason medicinal chemists and researchers love peptides is they offer a unique ability to treat unmet clinical needs in comparison to small molecules and biological therapeutics. With over 70 approved peptide therapeutics on the market treating patients today, researchers are looking for the next peptide therapeutic that will make waves.
What about synthesizing the peptides that researchers use for their studies? CSBio has been making peptides and peptide synthesizers for over 30 years, and we’ve developed synthesizers for the first-time peptide synthesis user as well as the advanced peptide chemist. Here’s the major things to consider when comparing research scale peptide synthesizers for your needs.
1. Manual or Fully Automated?
This day and age, a fully automated synthesizer is table stakes. But making sure the synthesizer you buy is fully automated is the most important factor.
For those that haven’t made a peptide before, a typical solid phase peptide synthesis protocol will consist of deprotection cycles, multiple washes, coupling, and more multiple washes for each amino acid addition to their peptide. Dependent on the peptide, there can be multiple washes and deprotection cycles to ensure adequate synthesis, defined by high purity of the targeted peptide being synthesized.
Typical Peptide Synthesis Cycle for each Amino Acid Addition:
- 2x Deprotection
- 6x DMF Wash
- Coupling
- 2x DMF Wash
When performing manual synthesis, the washes and deprotection cycles are extremely redundant and time consuming, requiring manual solvent addition and draining for each wash or deprotection cycle. Semi or manual peptide synthesizers typically only perform the coupling step, and require users to manually perform the remainder of the synthesis. Given the coupling step is the least labor intensive portion of the synthesis and accounts for less than 10% of the touch time, these peptide synthesizers are categorized as manual synthesizers.
What does it mean to have a fully automated peptide synthesizer? Most peptide sequences are around 20 amino acids long, and users would like to be able to setup and walk away from their peptide synthesizer and come back to a completed peptide. This means the synthesizer is able to fully automate the completion of a synthesis cycle for each amino acid, as well as continue to subsequent amino acids without any user intervention such as adding more solvents, reagents, or amino acids. Having a fully automated synthesizer that allows users to make an entire peptide without any manual steps in between is by far the most important factor to verify when buying a peptide synthesizer.
Take a look at our case study of the synthesis of a 132-mer peptide on CSBio’s research scale peptide synthesizers, which was performed entirely unattended without any solvent recharging, amino acid additions, or manual steps.
2. Reaction Vessel (RV) Size
The reaction vessel size determines the maximum synthesis scale that a peptide synthesizer is capable of performing, and synthesis scale determines the amount of peptide material that can be produced per synthesis batch. Most peptide synthesizers consider the solvent, reagent, and amino acid consumption based on the reaction vessel size to ensure adequate unattended automation; as users would not want a reaction vessel sized on a synthesizer that requires solvent addition every 30 minutes.
Dependent on what quantities (mg to grams) a user requires at their phase of research, determines the size of reaction vessel. The amount of peptide that can be produced in any given reaction vessel can depend on many factors including resin substitution, chemistry, number of amino acid additions, and expected growth. Some manufacturers only talk about synthesis scale based on mmol, but it’s difficult to compare when there are so many factors that determine mmol outside of reaction vessel size. To provide a rough idea, a 15ml reaction vessel can be used to produce anywhere from 50mg to 500mg dependent on these many factors. For a 20-mer peptide, using 0.4 mmol/gram substitution resin, a 0.1mmol synthesis can be performed with a 15ml reaction vessel producing ~300mg of crude peptide. Upon purification, dependent on purity requirements, this crude peptide can generally yield ~100mg of pure peptide.
3. Purity, Synthesis Speed, and Waste Generation
After determining the quantity of peptide required, a few other practical questions to ask when deciding on a peptide synthesizer is how many peptides will you make per month, and at what purity will you need for these peptides? Most research scale peptide labs are synthesizing around 10 peptides per month, and purity requirements can vary but 90 to 95% is typical. With these requirements, ensuing a high quality crude peptide leads to ease of purification.
What are the factors that drive a high quality crude peptide? It comes down to the peptide amino acid sequence, the length of the peptide, and the synthesis protocol. With an “easy” peptide, a fast protocol with low waste generation can yield a high quality crude peptide, while a “difficult” peptide with that same fast protocol can at times not even yield the correct target peptide (as identified by mass spectrometer) within the crude product.
The synthesis protocol itself is also the primary driver when it comes to synthesis speed and waste generation. As such, many times there’s a trade-off between synthesis speed, waste generation, and purity. Take a look at our article Demystifying Purity, Synthesis Speed and Waste Generation that talks in detail about this.
4. Chemistry Flexibility
For most users making peptides, the desired goal is to simply produce their target peptide, where having an easy to use peptide synthesizer is more desirable than having a lot of features which can increase flexibility, but also add complexity.
For the advanced peptide chemist, there may be a desire to have a lot of flexibility in performing different types of chemistry to improve synthesis yield, or to be able to develop a process for scale up. This flexibility could be performing double couplings, cappings, doing Fmoc synthesis, Tboc synthesis, using DIC coupling or HBTU coupling within the same peptide, to name a few.
While there are many detailed factors to consider that will drive this flexibility, primarily related to the software interface and how system functions are performed, a major factor is whether the system has the dedicated reagent bottles available. To perform DIC coupling on one amino acid addition, followed by HBTU coupling on the subsequent amino acid addition in the peptide sequence, requires the dedicated reagent bottles to be able to perform this in a fully automated manner.
These are the top things to consider when looking for a research scale peptide synthesizer. There are many other considerations as well, such as:
- Cost of the synthesizer, as well as cost of long term ownership when it comes to reliability and ease of repair and maintenance
- Performing chilled and heated synthesis
- Performing high throughput synthesis if a large quantity of peptides is required on a weekly basis.
- Scale up considerations, where users will desire to establish processes on research scale systems to move towards pilot and commercial scale peptide production.
Reach out to us atinstrument@csbio.com, schedule a call to learn more, or just ring us directly at +1 650 525 6200 if you’re interested in discussing your peptide synthesizer needs.
About CSBio: For over 30 years, CSBio, a leading peptide and peptide synthesizer manufacturing company located in Silicon Valley, California, has been providing cGMP peptides and automated peptide synthesizers to the global pharmaceutical community. CSBio’s peptide products and peptide synthesizers can be found in production laboratories, universities, and pharmaceutical companies worldwide.
Want to know when new
Resources are released? Be notified
Related Posts
Synthesizing a 132-mer peptide with high purity
Synthesis of peptides at any length requires extreme attention to detail for accuracy and purity of the desired compound...
Demystifying Purity, Synthesis Speed and Waste Generation
Understanding purity, synthesis speed, waste generation and finding that perfect peptide synthesis protocol for peptide synthesizers...
About Us
CSBio is a leading peptide and instrumentation manufacturing company located in Silicon Valley, California.
CSBio provides nonGMP and cGMP peptides, peptide APIs, research scale peptide synthesizers, commerical scale peptide synthesizers, DNA/RNA oligonucleotide synthesizers, and preparative HPLC purification equipment.
Useful Links
Instrumentation Contact
915 Linda Vista Ave
Mountain View, CA, 94043
+1-650-525-6200